Investigation of a technique
for safely storing secondary waste generated
by the processing of
contaminated water

Interviewee
Dr Isao Yamagishi
Research purpose and contents
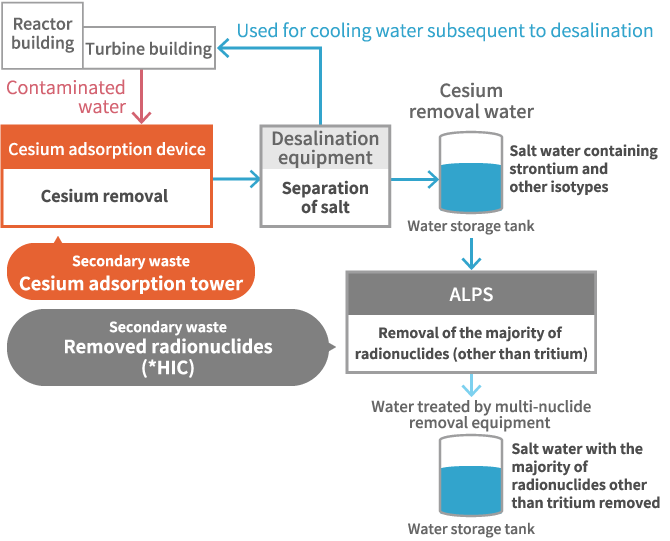
Contaminated water with high concentrations of radionuclides are processed in a cesium adsorption tower and multi-isotype removal facility [hereafter, ALPS (Advanced Liquid Processing System) ]. The majority of radionuclides other than tritium are removed; however, used adsorption materials generated in this process and the removed radionuclides are generated as secondary wastes. To implement decommissioning measures, the establishment of a technique for the management of radioactive waste (storage, processing, and disposal) is essential. In this study, our research focuses on the storage of secondary waste.
Investigation of the long-term storage policy for the cesium adsorption tower
The cesium adsorption device is first used on contaminated water that contains radioactive materials discharged from reactor core buildings as well as turbine buildings. The device removes cesium and strontium, which comprise the majority of the radioactive materials contained in the contaminated water. Zeolite is used as an adsorbent; however, when it adsorbs a fixed amount of cesium, it is no longer able to adsorb anymore. Therefore, cesium adsorption towers that adsorb radioactive materials are regularly replaced and stored.
Purification treatment of decontaminated water
As the adsorption tower initially treats contaminated water, including sea water, it is critical
to estimate the corrosion status of the adsorption tower, which is a stainless steel container,
and the properties of the adsorbent material, and furthermore, assess the generation of hydrogen
there as well as the vessel corrosion.With CLADS, it is possible to perform full-scale
(non-radioactive) tests and analyze changes in the waste properties for long-term storage.
Using simulated zeolite adsorbent materials and cesium adsorption tower test samples, we
performed a long-term storage test, wherein a heater was used to simulate the generation of heat
based on cesium radiation. The biggest issue with respect to storage is the generation of
hydrogen and corrosion, and these are both caused by the presence of water. Therefore, if the
water and damp absorbents, which remain in the adsorption tower, are have completely dried, the
risks of storage are greatly reduced.However, naturally drying them may take several decades.
Moreover, there is a concern that during the drying process, the salinity of the water will
increase, thereby enhancing the risk of corrosion. In addition, as it is not possible to open
the lid of the container when sampling is difficult, small-scale tests and actual scale
tests(non-radioactive) play an important role in estimating the state inside the container. We
have developed a program for analyzing and predicting the drying out process for absorbent
materials.
 Appearance of cesium adsorption tower test sample
Appearance of cesium adsorption tower test sample
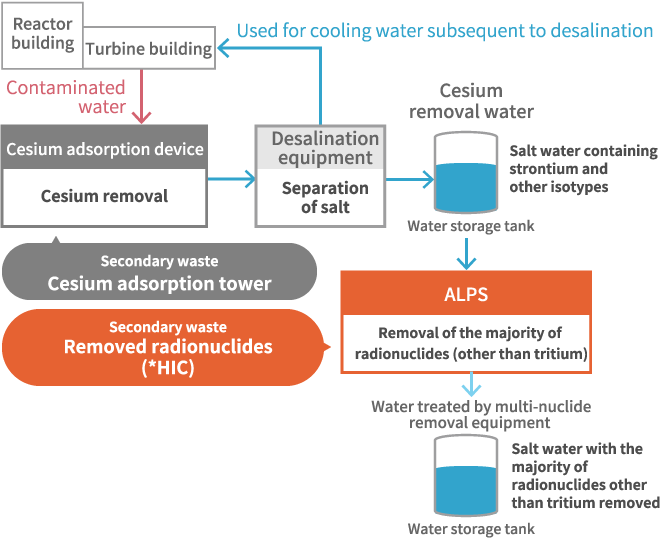
HIC for multiple isotype removal facility
With ALPS, it is possible to eliminate 62 types of radioactive materials from contaminated water from which cesium has already been eliminated utilizing a cesium adsorption device. The removed radioactive waste is stored in an HIC (High Integrity Container) and stored subsequent to being transported to a temporary storage facility.
Purification treatment of decontaminated water
Within the HIC, there were many items containing thick liquids, known as slurries, and there was a water overflow problem from the HIC during storage. This could be attributed to the radiation emitted by strontium present in the slurry, causing water to decompose and leading to the formation of hydrogen foam. We performed a simulation test to evaluate this phenomenon and confirmed the expansion of the slurry and the increase in the liquid surface as a consequence of radiation exposure. We are currently working to elucidate the detailed mechanisms with respect to the conditions that lead to the water overflowling and the mechanism of the hydrogen foam generation.
Polymers that harden and stabilize the zeolite
In addition, in relation to processing than storage, we are also investigating the basic technology for solidification and stabilization, including the underlying water itself. Using simulated zeolite adsorbent materials and cesium adsorption tower test samples, we performed a long-term storage test, wherein a heater was used to simulate heat generation based on cesium radiation. Zeopolymers exhibit excellent heat resistance and cesium holding abilities and are being investigated as a potential measure even for hydrogen, which is associated with long-term storage issues.
Aim for a more logical management
At present, contaminated water treatment tanks comprise the majority of the available space in decommissioning. By treating the contaminated water, the secondary waste products generated will continue to increase, and there have been demands for reductions and decreases in the generated volume. However, by compacting, the density increases and this results in a variety of risks. In addition, the current storage method is a method of proceeding based on the status of the accident response and decommissioning measures. This needs to be reviewed. Our research and investigation in the future will aim for a safer and more logical method of storage.
Related information
| References | 1…I.Yamagishi, et al. “Difficulties in Treatment of Contaminated Water in Fukushima-1
Nuclear Power Plant and Disposal of Its Secondary Waste” Journal of the Atomic Energy
Society of Japan, 54(3), pp.166-170 (2012). 2…I.Yamagishi, et al., “Characterization and storage of radioactive zeolite waste” , J. Nucl. Sci. Technol., 51(7-8), pp.1044-1053 (2014). 3…I.Yamagishi, et al. “Study on wet waste storage—irradiation behavior of carbonate slurry and Wet Zeolite”, Conference paper, 3rd International Forum on th0.e Decommissioning of the Fukushima Daiichi Nuclear Power Station. Iwaki, Japan (2018. Available from https ://ndf-forum.com/3rd/en/ 4…V.Cantarel, et al., “On the hydrogen production of geopolymer wasteforms under irradiation”, J.Am.Ceram.Soc, 102, pp.7553-7563 (2019). |
|---|